MEDIUM
Earn 100
For an ionic micelle-forming surfactant near its critical micelle concentration (CMC), the dependence of molar conductivity and surface tension on surfactant concentration is best represented by,
(a)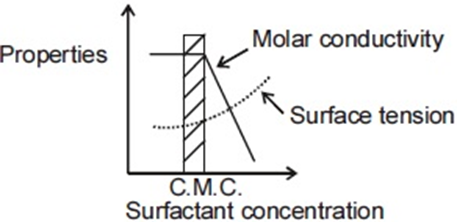

(b)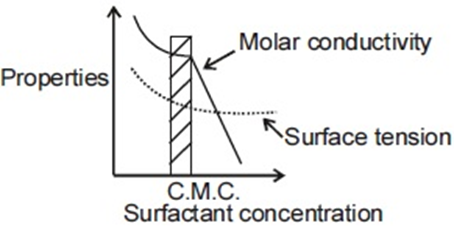

(c)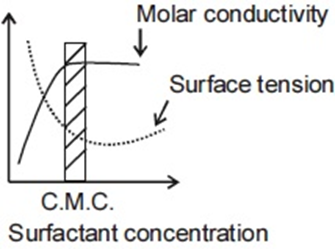

(d)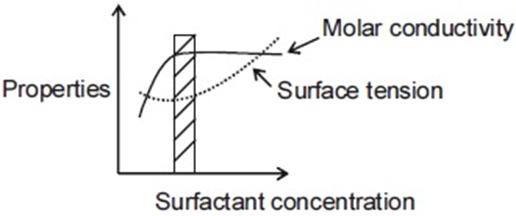

50% studentsanswered this correctly
Important Questions on Surface Chemistry
EASY
MEDIUM
Identify the correct molecular picture showing what happens at the critical micellar concentration of an aqueous solution of a surfactant

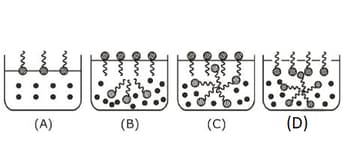
EASY
EASY
MEDIUM
(Critical micelle concentration (CMC) is marked with an arrow in the figures.)
EASY
Match the following:
| List- | List- | ||
| Type of Colloid | Phase in medium | ||
| () | Aerosol | () | Solid in solid |
| () | Emulsion | () | Liquid in solid |
| () | Foam | () | Gas in liquid |
| () | Gel | () | Solid in gas |
| () | Liquid in liquid |
The correct answer is
EASY
EASY
EASY
EASY
Match the following:
| (i) Foam | (a) smoke |
| (ii) Gel | (b) cell fluid |
| (iii) Aerosol | (c) jellies |
| (iv) Emulsion | (d) rubber |
| (e) froth | |
| (f) milk |
EASY
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM

