For next two question please follow the same
The figure shows a V - T graph of process carried on one mole of mono atomic gas. The slope of the graph in the process CA varies as where is a constant and p is the pressure of the gas.
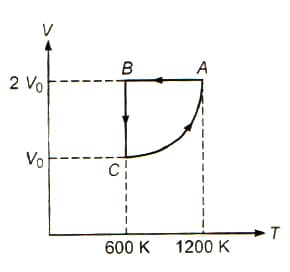
The relation between temperature and pressure variation for the process CA is

Important Questions on Kinetic Theory
( is universal gas constant and is the acceleration due to gravity)
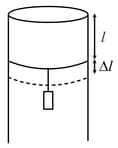
A long cylindrical pipe of radius is closed at its upper end and has an airtight piston of negligible mass as shown. When mass is attached to the other end of piston, it moves down by a distance, before coming to equilibrium. Assuming air to be an ideal gas, (see figure) is close to , one atmospheric pressure is ),
An ideal gas undergoes a circular cycle centred at , as shown in the diagram.
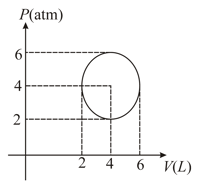
The maximum temperature attained in this process is close to
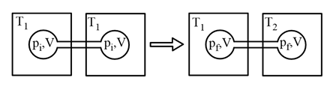
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?

