EASY
JEE Main
IMPORTANT
Earn 100
For the compounds CH3Cl, CH3Br, CH3I and CH3F, the correct order of increasing C-halogen bond length is :
(a)CH3F < CH3Br < CH3Cl < CH3I
(b)CH3F < CH3I < CH3Br < CH3Cl
(c)CH3F < CH3Cl < CH3Br < CH3I
(d)CH3Cl < CH3Br < CH3F < CH3I
68.22% studentsanswered this correctly

Important Questions on Haloalkanes and Haloarenes
MEDIUM
JEE Main
IMPORTANT
In a nucleophilic substitution reaction : , which one of the following undergoes complete inversion of configuration ?
HARD
JEE Main
IMPORTANT
The decreasing order of reactivity towards dehydrohalogenation reaction of the following compounds is:
MEDIUM
JEE Main
IMPORTANT
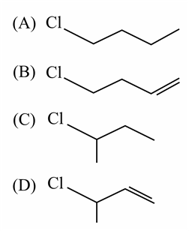
The major product of the following reaction is:
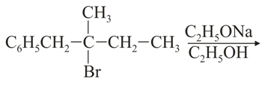
EASY
JEE Main
IMPORTANT
The number of chiral carbons in chloramphenicol is ____________.
MEDIUM
JEE Main
IMPORTANT
Compound (A), , gives a white precipitate when warmed with alcoholic . Oxidation of (A) gives an acid (B), . (B) easily forms anhydride on heating. Identify the compound (A).
EASY
JEE Main
IMPORTANT
The correct statement for the molecule , is :
MEDIUM
JEE Main
IMPORTANT
In SN2 reactions, the correct order of reactivity for the following compounds :
CH3Cl, CH3CH2Cl, (CH3)2CHCl and (CH3)3CCl is :
HARD
JEE Main
IMPORTANT
The increasing order of the boiling points of the major products and of the following reactions will be :
(a)
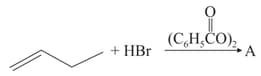
(b)
(c)