MEDIUM
Earn 100
For the following equilibrium in a closed container
At a constant temperature, the volume of the container is halved suddenly, which of the following is correct for and ?
(a)Both and changes.
(b)Neither nor change.
(c) changes but does not.
(d) changes but does not.
50% studentsanswered this correctly
Important Questions on Chemical Equilibrium
MEDIUM
MEDIUM
MEDIUM
Using the data provided, find the value of equilibrium constant for the following reaction at and atm pressure.
MEDIUM
MEDIUM
When and are compared at It is found that
MEDIUM
EASY
At equilibrium, the mass of each of the reactants and products remains constant.
At equilibrium, the rate of forward reaction is equal to the rate of backward reaction.
HARD
The reaction
is in equilibrium in a closed vessel at The partial pressure (in atm) of in the reaction vessel is closest to:
[Given: The change in Gibbs energies of formation at and bar for
Gas constant ]
EASY
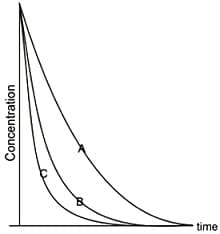
These profiles imply that the decay constants and follow the order
EASY
EASY
The rate of this reaction is increased by
MEDIUM
The value of for the reaction
The value of for the following reaction is :
MEDIUM
Find equilibrium constant for given condition: starting from of in container, of dissociates till equilibrium at .
EASY
HARD
EASY
EASY
EASY
EASY
MEDIUM

