MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
For the following first order gaseous reaction
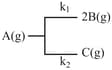
The initial pressure in a container of capacity litres is . Pressure at time is and after infinite time it becomes atmosphere. Find the rate constant and for the appropriate reactions.

50% studentsanswered this correctly

Important Questions on Chemical Kinetics
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
