EASY
Earn 100
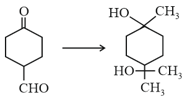
For the following reaction, identify the carbonyl compound that shows the highest reactivity.

(a)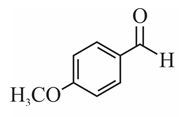

(b)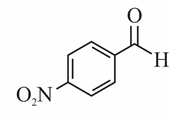

(c)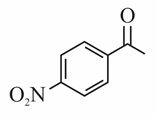

(d)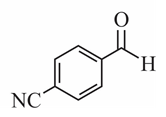

100% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
EASY
In an reaction, the group that leaves the substrate is called a leaving group. The ease of departure of leaving group is determined on the basis of acidity of the conjugate acid of the leaving group. The stronger the conjugate acid, the better is the leaving group. The compound having the best leaving group is
EASY
In electrophilic aromatic substitution reactions of chlorobenzene, the ortho/para-directing ability of chlorine is due to its
MEDIUM
The correct order of nucleophilicity is
EASY
Consider the following compounds
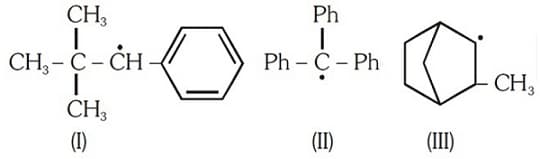
Hyperconjugation occurs in:
MEDIUM
The increasing order of nucleophilicity of the following nucleophiles is;
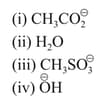
HARD
A solution of in toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of :
HARD
What will be the correct nucleophilicity order in protic or aprotic solvents?
MEDIUM
Which of the following molecules is least resonance stabilized?
EASY
Which among the following is a set of nucleophiles?
EASY
The indicated atom is not a nucleophilic site in
MEDIUM
In which of the following compounds, the bond ionization shall give most stable carbonium ion?
EASY
Which of the following statements is not correct for a nucleophile?
EASY
In pyrrole, the electron density is maximum on
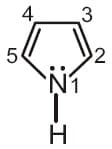
EASY
The order of stability of the following carbocations:
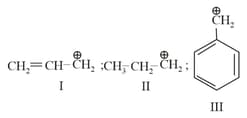
MEDIUM
In which of the following molecules, all atoms are coplanar?
MEDIUM
The stability of carbocations

follows the order
MEDIUM
The hyperconjugative stabilities of tert-butyl cation and 2-butene, respectively, due to
MEDIUM
Given below are two statements :
Statement I : and both can generate nucleophile.
Statement II : and both will generate nitrile nucleophile with all reaction conditions.
Choose the most appropriate option :
EASY
The correct statement regarding electrophiles is:
MEDIUM
The correct sequence of reagents for the following conversion will be