HARD
JEE Main/Advance
IMPORTANT
Earn 100
For the reaction , the experimental data require the following rate equation:
Which of the following is/are true regarding this?
(a)The reaction is a single step reaction.
(b)The reaction is order in the initial stages
(c)The reaction is order in the final stages .
(d)The molecularity of the reaction is two.
100% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
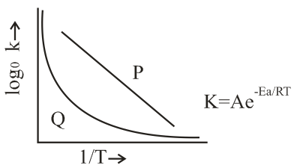
Which of the following statements are true regarding the plot shown in the given diagram?
EASY
JEE Main/Advance
IMPORTANT
If the rate of reaction is given by: Rate
Which statements are correct?
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
