Formation of magnesium oxide is an example of displacement reaction.
Important Questions on Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved.
A metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle.
Write a balanced equation for the reaction if one of the compounds formed is calcium chloride.
Choose the method of preparation of Iron (III) chloride, from the methods given in the list:
[List: A. Neutralization B.Precipitation C.Direct combination D.Substitution]
This reaction is
Choose the method of preparation of Iron (II) sulphate, from the methods given in the list:
[List: A. Neutralization B.Precipitation C.Direct combination D.Substitution]
Which of the following are combination reaction?
(i)
(ii)
(iii)
(iv)
Match the items of the column -I with column-II and choose the correct option.
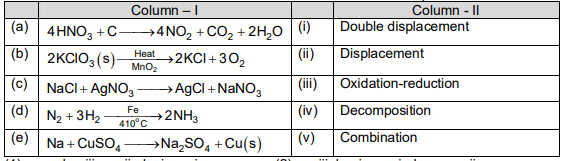
Which of the following is a reversible reaction?
| List- (Chemical reactions) | List- (Type of chemical reactions) |
| Addition | |
| Elimination | |
| Redox | |
| Substitution |
Consider the following equation of the chemical reaction of a metal M
The equation represents:-
Calcium oxide reacts vigorously with water to produce slaked lime.
This reaction can be classified as:
(A) Combination reaction (B) Exothermic reaction
(C) Endothermic reaction (D) Oxidation reaction
Which of the following is the correct answer?

