MEDIUM
Earn 100
Formation of which complex, among the following, is not a confirmatory test of ions
(a)Lead sulphate
(b)Lead nitrate
(c)Lead chromate
(d)Lead iodide
46.43% studentsanswered this correctly
Important Questions on Inorganic Qualitative Analysis
MEDIUM
MEDIUM
State one relevant observation for of the following:
Lead nitrate solution is treated with sodium hydroxide solution dropwise till it is in excess.
HARD
HARD
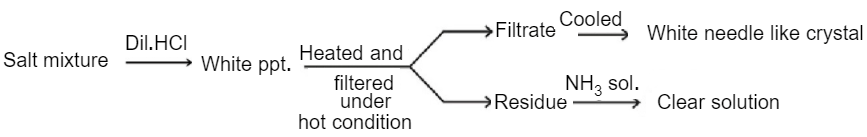
A mixture of two salts is used to prepare a solution which gives the following results:
The correct option for the salt mixture is(are)
EASY
MEDIUM
MEDIUM
HARD
What are the cations present in the salt ?
HARD
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
EASY
Therefore is
HARD
A solution of a salt gives a precipitate on treatment with dilute HCl. The precipitate formed is filtered off and treated with NH3 whereby it turns black. The cation present in the salt is
MEDIUM
MEDIUM
Balance the chemical equation

