MEDIUM
Earn 100
Formic acid is a stronger acid than acetic acid. This can be explained using
(a)effect
(b) effect
(c) effect
(d) effect
50% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
The compound that does NOT liberate CO2, on treatment with aqueous sodium bicarbonate solution, is
HARD
The correct order of acidity for the following compounds is :
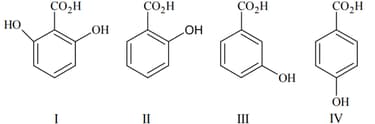
EASY
The weakest among the following acids is
EASY
The correct increasing order of the basic strength for the following compounds is:
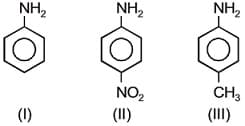
MEDIUM
The correct order for acid strength of compounds and is as follows:
EASY
Among formic acid, acetic acid, propanoic acid and phenol, the strongest acid in water is
MEDIUM
The correct order of acidity of the following compounds is

EASY
The which contribute least to the basicity of the compound is :
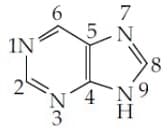
MEDIUM
The correct statement regarding the basicity of aryl amines is:
HARD
The correct order of strengths of carboxylic acids is
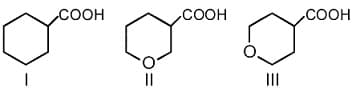
MEDIUM
Considering the basic strength of amines in an aqueous solution, which one has the smallest value?
MEDIUM
The increasing basicity order of the following compounds is:
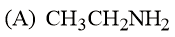
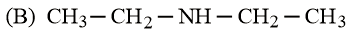
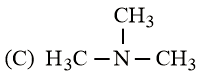
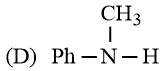
EASY
Among the following oxoacids, the correct decreasing order of acid strength is :
EASY
The acidity of compounds in water
. Ethanol . Acetic Acid . Phenol . Acetonitrile
follows the order
HARD
The increasing order of basicity of the following compounds is :
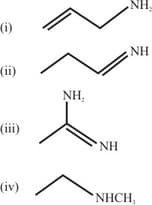
HARD
The increasing order of the of the following compund is:
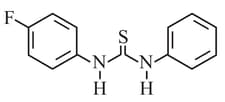
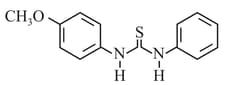
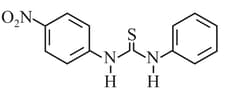
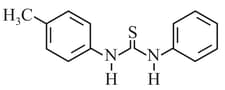
HARD
What is the order of basicity among the following compounds?
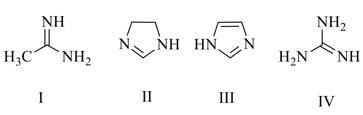
EASY
In case of substituted aniline the group which decreases the basic strength is
MEDIUM
Among the following, which is least acidic?
MEDIUM
The correct order of basicity is

