HARD
JEE Main
IMPORTANT
Earn 100
Fraction of molecules are plotted against velocity of molecules, according to Maxwell-Boltzmann distribution law. The velocity corresponding to the points and are:
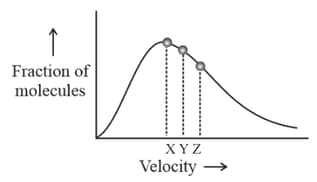
Here,
most probable velocity.
average velocity.
root mean square velocity.

average velocity.
root mean square velocity.
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on States of Matter
HARD
JEE Main
IMPORTANT
Which of the following facts regarding an ideal gas is/are correct?
MEDIUM
JEE Main
IMPORTANT
Which of the following statements are incorrect?
MEDIUM
JEE Main
IMPORTANT
Which of the following statements are correct?
HARD
JEE Main
IMPORTANT
Let , and , respectively denote the average speed, root mean square speed and most probable speed in an ideal monatomic gas at Kelvin temperature . The mass of an atom is . Then,
HARD
JEE Main
IMPORTANT
Determine the volume correction and pressure correction for kept in flask. Given and for .
HARD
JEE Main
IMPORTANT
The value of '' for steam is . The density of liquid water is at . What percentage of volume of water molecules occupy in gaseous phase of water in liquid phase?
HARD
JEE Main
IMPORTANT
Determine the pressure exerted by of in vessel at using van der Waals equation. Also report the pressure of gas, if it behaves ideally in nature. Given .
MEDIUM
JEE Main
IMPORTANT
Calculate the compressibility factor for carbon dioxide, if one mole of it occupies at and . Comment on the result.
