EASY
NEET
IMPORTANT
Earn 100
Freezing up liquid in a system then:
(a)
(b)
(c)
(d) or (depending on the nature of liquid)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Five moles of a gas is put through a series of changes as shown graphically in a cyclic process the , and , respectively are
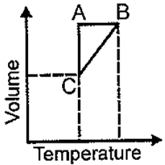
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
An ideal gas is taken around the cycle as shown in diagram. The net work done by the gas during the cycle is equal to:
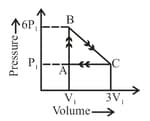
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
