MEDIUM
Earn 100
Give a reason for the following:
Sodium and potassium are stored in kerosene.
Important Questions on Water
EASY
MEDIUM
Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
EASY
MEDIUM
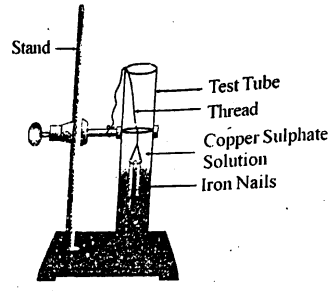
Write a chemical equation for the reaction taking place in the test tube. How does the colour of the solution change? What is the change in colour of iron nails?
EASY
HARD
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
Give reasons-
Why copper is used to make hot water tanks and not steel (an alloy of iron)?
HARD
State whether the Solution formed by the reaction of sodium with water is acidic or basic.
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
HARD

