EASY
Earn 100
Give an example for a first order reaction.
Important Questions on Chemical Kinetics
EASY
It takes for a first order reaction to go to completion. The total time required for the same reaction to reach completion will be
EASY
One gram of () decays by -emission to in years. The half-life period of the reaction is
EASY
, when is doubled keeping constant rate increases times, when is constant and is doubled, rate increases four times. The overall order is
EASY
What are elementary reactions?
EASY
The reaction is of the first-order. If the volume of the reaction vessel is reduced to the rate of the reaction would be
EASY
A first order reaction takes minutes for completion. Calculate the half life of reaction.
EASY
In a chemical reaction products, when concentration of is doubled, rate of the reaction increases times and when concentration of alone is doubled rate continues to be the same. The order of the reactions is
MEDIUM
The rate of a reaction is found to depend upon two concentration variables. What should be the order of the reaction?
MEDIUM
For the chemical reaction
, the reaction proceeds as follows
(Fast)
, (Slow)
the rate law expression should be given as
EASY
The rate constant is same for reactions of order , and , respectively, the unit of concentration being in moles per litre. If the concentration of the reactant is unity, the rates of reaction will be
EASY
The units for the rate constant and the rate of reaction are same for a reaction. What will be the order of the reaction?
MEDIUM
Decomposition of follows a first order reaction. In fifty minutes the concentration of decreases from to in one such decomposition. When the concentration of reaches , the rate of formation of will be:
EASY
Hydrolysis of ester is a first order reaction. For this reaction, the correct statement is
HARD
Gaseous cyclobutene isomerizes to butadiene in a first order process which has a '' value of at The time in minutes it takes for the isomerization to proceed to completion at this temperature is_____. (Rounded off to the nearest integer)
HARD
What will be the order of the reaction for hydrolysis of methyl acetate with by using the data provided?
Time
Volume of acid
MEDIUM
Graph plotted between versus log concentration is a straight line. What conclusion can you draw from the graph?
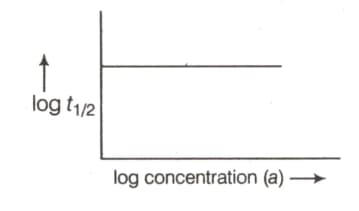
order of reaction
EASY
For a certain reaction between and the order with respect to is and that with respect to is If the concentrations of both and are tripled. the rate of reaction will increase by a factor of ________
MEDIUM
The time required for completion of a first order reaction is . The time required for completion of the same reaction will be
EASY
For the elementary reaction , the rate of disappearance of increases by a factor of upon doubling the concentration of . The order of the reaction with respect to is
HARD
In the reaction,
the time taken for reaction of is twice the time taken for reaction of . The concentration of varies with reaction time as shown in the figure.The overall order of the reaction is: