MEDIUM
Earn 100
Give reasons.
Some elements have variable valencies.
Important Questions on Measurement of Matter
EASY
EASY
MEDIUM
MEDIUM
HARD
Draw the electron dot diagram for the compound given below. Represent the electrons by (.) and (x) in the diagram.
- Water molecule [Atomic No. of ]
HARD
MEDIUM
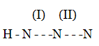
The above compound represents hydrogen azide, the bond orders of bonds and are:
EASY
MEDIUM
Draw the electron dot diagram of Hydronium ion.
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM

