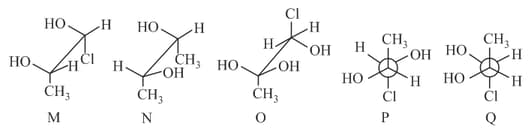
Give the Newman projection formula of the least stable staggered form of butane. Which of the following reasons is the causes of its unstability?
() Vander Waals strain
() Torsional strain
() Combination of both

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
Newman projection of butane is given. If is rotated by along and bond in the anticlockwise direction, the conformation formed is
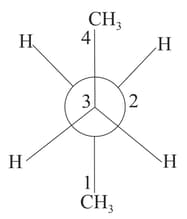
It is given that for conformational isomers, the net dipole moment is
Where,
Observed dipole moment of the compound
Dipole moment of the stable conformational isomers
Mole fraction of stable conformers
for the compound , draw the Newman projection formula of all the stable conformational isomers, if , , and find . Now, draw the Newman projection formula of the most stable conformation of meso .
(a) If is , (rotation about bond)
(b) If is , (rotation about bond)
The correct statement(s) about the compound given below is/are
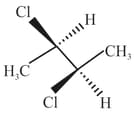
The correct statement(s) about the compound
is (are)
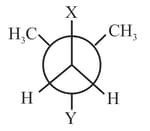
In Newman projection, for dimethylbutane, and can respectively be
The number of optically active products that are obtained from the complete ozonolysis of the given compound are :-
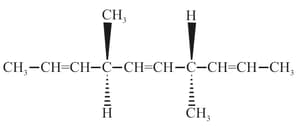
Which of the given statements about and with respect to is/are correct?