EASY
Earn 100
Give the order of ease of decarboxylation of the following acids
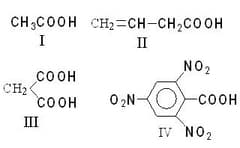
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Aldehydes, Ketones and Carboxylic Acids
MEDIUM
Which one of the following carboxylic acids decarboxylate easily?
EASY
The reagent which can do the conversion is
HARD
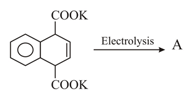
The molecular formula of the final product of the following reaction sequence is _________
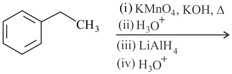
EASY
Explain the following reactions with equations: Decarboxylation.
MEDIUM
How will you bring about the following conversion in not more than two steps?
Benzoic acid to m-Nitrobenzyl alcohol
EASY
What is the reagent 'A' used in the following equation?
MEDIUM
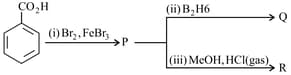
The major products and from the following reactions, respectively are
EASY
What happens when acetic acid is reduced by lithium aluminium hydroxide, and what happens when acetic acid is treated with ammonium hydroxide and the resulting product is heated at high temperature?
MEDIUM
Which of the following acid will form an (a) Anhydride on heating and (b) Acid imide on strong heating with ammonia?
MEDIUM
Predict the product:
HARD
In the reaction,
,
the product C is :
MEDIUM
Reduction of cinnamic acid with Gives the following
EASY
How will you convert sodium acetate to methane?
EASY
What happens when (Write chemical equations only) Acetic acid is heated with ?
EASY
Describe the following :
Decarboxylation.
EASY
How will you obtain Formic acid from oxalic acid? (Give chemical equations only).
MEDIUM
Describe the following:
Decarboxylation
MEDIUM
chlorosodium acetate on boiling with aqueous sodium nitrite gives
EASY
Give the chemical equation for the reaction of with following: