Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: In equation value of depends on .
Reason R: is an intensive property and is an extensive property.
In the light of the above statements, choose the correct answer from the options given below:
Reason R: is an intensive property and is an extensive property.
Important Questions on Redox Reactions and Electrochemistry
is
The emf of the following cell is at .
The standard free energy change for the cell reaction is________
The standard electrode potential and its temperature coefficient for a cell are and at , respectively. The reaction is . The standard reaction enthalpy at in is
Use and
For the cell reaction
at The standard Gibbs energy of the cell reaction is:
[Given that Faraday constant ]
The standard emf of the cell and equilibrium constant of the following reaction of
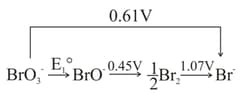
The value of is
at is approximately:
Given that:
The standard e.m.f. of the cell, in which the cell reaction is is at and at . The enthalpy change of the reaction at is
at for the cell is The equilibrium constant at is
According to the expression , the cell reaction is spontaneous when
(Notations and symbols carry their usual meanings)

