Given below is the balanced chemical equation for the thermal decomposition of lead nitrate.
Which of the following information does the coefficients of and in the equation ( and respectively) tell us?

Important Questions on Chemical Reactions and Equations
The diagram below shows the set-up in which the electrolysis of water takes place.
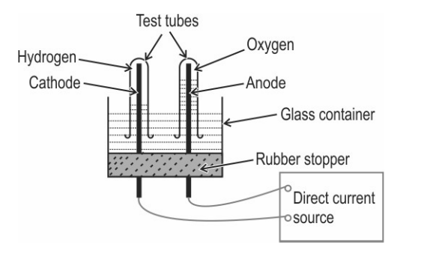
What type of reaction takes place?
The diagram below shows the set-up in which the electrolysis of water takes place.
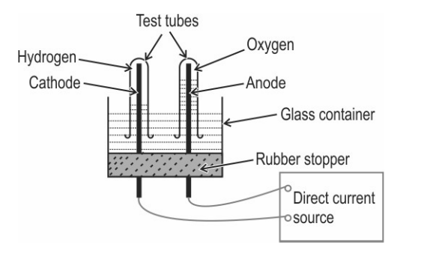
Explain why this is an example of an endothermic reaction?
The diagram below shows the set-up in which the electrolysis of water takes place.
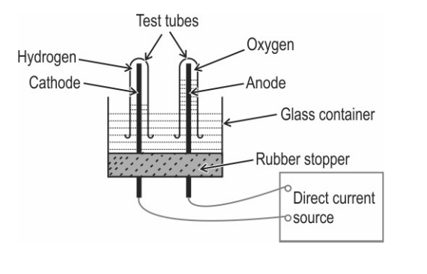
The test tube containing hydrogen is removed carefully from the apparatus. A lit match stick is brought near the mouth of this test tube. The gas burns with an explosive "pop" sound.
Write a balanced chemical equation for the above electrolysis reaction and indicate whether energy is absorbed or released.
Eight identical, iron blocks are placed on the ground in the two arrangements X and Y as shown below. The block arrangements are kept moist by sprinkling water every few hours.
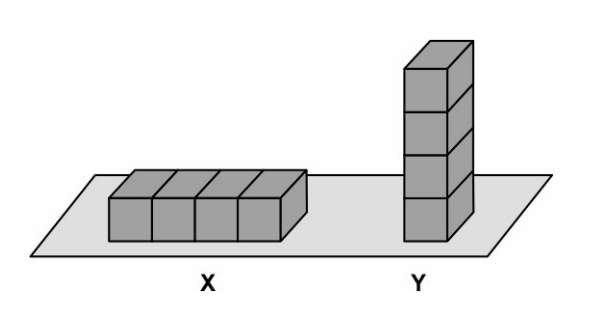
Which of the arrangements is likely to gather more rust after ten days? justify your answer.
The following chemical equation does not represent a chemical reaction that can take place.
State what needs to be changed in the equation above for it to represent the correct reaction between and .
Trupti mixes an aqueous solution of sodium sulphate and an aqueous solution of copper chloride
Will this lead to a double displacement reaction? justify your answer.
Dilip was comparing combination reactions with decomposition reactions.
Which class of chemical substances may be the product of a decomposition reaction but NOT a product of a combination reaction?
