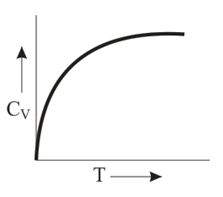
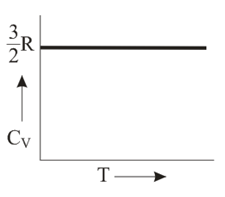
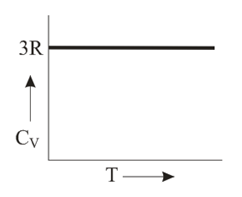
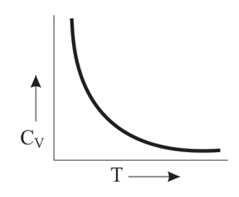
Graph of heat capacity at constant volume for a monoatomic gas is
Important Questions on Thermodynamics
The quantity of heat (in ) required to raise the temperature of of ethanol from to the boiling point and then change the liquid to vapor at that temperature is closest to [Given, boiling point of ethanol . Specific heat capacity of liquid ethanol . Latent heat of vaporisation of ethanol ]
(a) Internal energy and enthalpy each depends on temperature.
(b) Compressibility factor is not equal to 1
(c)
(d) for any process
[Heat of fusion of ice ; Specific heat of water ]
of is mixed with of The molar heat of neutralization of this reaction is The increase in temperature in of the system on mixing is The value of is (Nearest integer)
[Given: Specific heat of water
Density of water]
(Assume no volume change on mixing)
The molar heat capacity for an ideal gas at constant pressure is . The change in internal energy is upon heating it from to . The number of moles of the gas at constant volume is____
(Given: )
The specific heat of a certain substance is . Assuming ideal solution behavior, the energy required (in ) to heat of molal of its aqueous solution from to is closest to :
[Given: molar mass of the substance ; specific heat of water ]

