HARD
Upper Secondary: IGCSE
IMPORTANT
Earn 100
Gypsum is insoluble in water. You are asked to purify a sample of gypsum that is contaminated with a soluble salt. Write step-by-step instructions for the procedure.

Important Questions on Separating Substances
EASY
Upper Secondary: IGCSE
IMPORTANT
Explain the term in your own words:
Soluble
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
Argon, oxygen, and nitrogen are obtained from air by fractional distillation. Liquid air at , is warmed up and the gases collected one by one. Is liquid air a mixture, or a pure substance?
HARD
Upper Secondary: IGCSE
IMPORTANT
Argon, oxygen and nitrogen are obtained from air by fractional distillation. Liquid air at, is warmed up and the gases collected one by one. Explain why fractional distillation is used rather than simple distillation.
HARD
Upper Secondary: IGCSE
IMPORTANT
Argon, oxygen and nitrogen are obtained from air by fractional distillation. Liquid air at , is warmed up and the gases collected one by one. During the distillation nitrogen gas is obtained first, then argon and oxygen. What can you say about the boiling points of these three gases?
HARD
Upper Secondary: IGCSE
IMPORTANT
A mixture of salt and sugar has to be separated, using the solvent ethanol. Draw a diagram to show how you will separate the salt.
HARD
Upper Secondary: IGCSE
IMPORTANT
A mixture of salt and sugar has to be separated, using the solvent ethanol. How could you obtain sugar crystals from the sugar solution, without losing the ethanol?
HARD
Upper Secondary: IGCSE
IMPORTANT
A mixture of salt and sugar has to be separated, using the solvent ethanol. Draw a diagram of the apparatus used in the experiment of obtaining sugar crystals from the sugar solution, without losing the ethanol.
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
The diagram below shows a chromatogram for a mixture of amino acids.
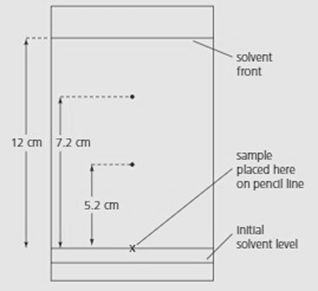
| Amino acid | value |
| Cysteine | 0.08 |
| Lysine | 0.14 |
| Glycine | 0.26 |
| Serine | 0.27 |
| Alanine | 0.38 |
| Proline | 0.43 |
| Valine | 0.60 |
| Leucine | 0.73 |
The solvent was a mixture of water, butanol and ethanoic acid. How will the values change if the solvent travels only cm?
