gas is adsorbed on activated charcoal to a very little extent in comparison to easily liquefiable gases due to _____.
Important Questions on Surface Chemistry
Four gases, and have critical temperatures and respectively
For their adsorption on a fixed amount of charcoal, the correct order is :
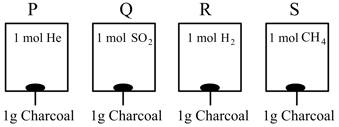
From the figure, in which of the following vessel, the pressure of the gas is the highest. [Temperature and volume of the gases are the same in each vessel].
Given below are two statements. One is labelled as Assertion and the other is labelled as Reason .
Assertion : Activated charcoal adsorbs more efficiently than .
Reason : Gases with lower critical temperatures are readily adsorbed by activated charcoal. In the light of the above statements, choose the correct answer from the options given below.
Adsorption of the gas increases with:
Predict which of the following gases show least adsorption on a definite amount of charcoal? (Critical temperature) of gases in scale is as

