Haloalkanes contain functional group where is a halogen. Present all the possible structural isomers of a substituted alkane with the molecular formula . State the IUPAC name of the compounds that you have presented.

Important Questions on Does Organic Chemistry Mean We Can Make Any Substance We Want?
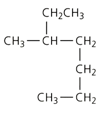
Apply the IUPAC system and deduce the name of the branched chain alkane.
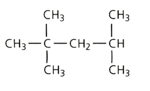
Apply the IUPAC system and deduce the name of the following branched chain alkane.
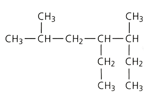
Apply the IUPAC system and deduce the names of the following branched chain alkane:
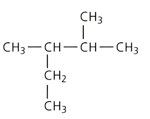
Apply the IUPAC system and deduce the names of the following branched chain alkane:
Consider the following data of the melting point of the first ten alkanes:
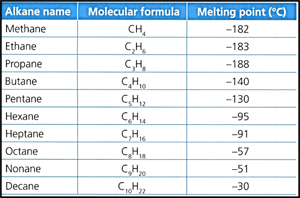
Identify, with reference to the information in the data table, how many degrees colder than ice, which melts at , is the melting point of methane.
Consider the following data of the melting point of the first ten alkanes:
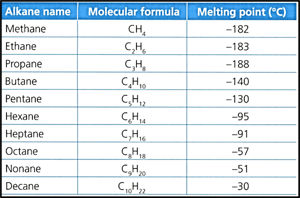
Identify, with reference to the information in the data table, how many degrees colder than ice, which melts at , is the melting point of decane.
Consider the following data of the melting point of the first ten alkanes:
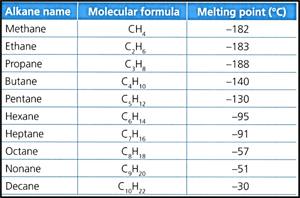
Identify, with reference to the information in the data table, the relationship between the length of alkane chain and its melting point.
Consider the following data of the melting point of the first ten alkanes:
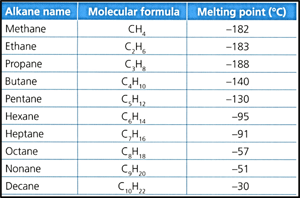
Identify, with reference to the information in the data table, what can you deduce about intermolecular attractions in decane compared to with those in methane.
