MEDIUM
Earn 100
How Langmuir adsorption isotherm is different from Freundlich adsorption isotherm?
Important Questions on Surface Chemistry
MEDIUM
Consider the following statements about Langmuir isotherm:
The free gas and adsorbed gas are in dynamic equilibrium.
All adsorption sites are equivalent.
The initially adsorbed layer can act as a substrate for further adsorption.
The ability of a molecule to get adsorbed at a given site is independent of the occupation of neighbouring sites.
The correct statements are:
HARD
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
HARD
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
HARD
HARD
From the following graphs, which two represent Langmuir adsorption isotherms?
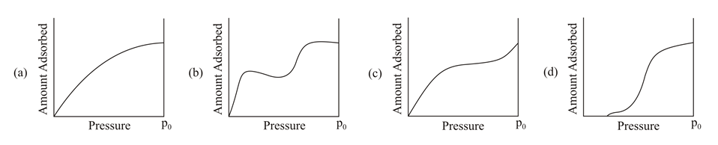
Select the correct option.
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM

