MEDIUM
Earn 100
How does carboxylate ion get stabilised by resonance? Explain by the structure.
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
The higher stabilities of tert-butyl cation over isopropyl cation and trans-- butene over propene, respectively, are due to orbital interactions involving
MEDIUM
Which of the following represents the hyperconjugation effect?
EASY
The chlorine atom of the following compound
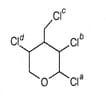
that reacts most readily with to give a precipitate is
MEDIUM
Arrange the following compounds in order of decreasing acidity :
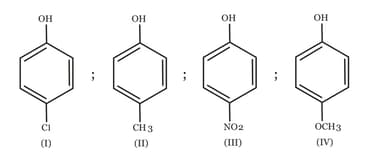
MEDIUM
Which of the following molecules is least resonance stabilized?
EASY
Which of the following compound is the most basic?
EASY
The most acidic proton and the strongest nucleophilic nitrogen in the following compound
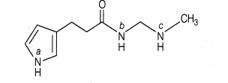
respectively, are
EASY
The order of stability of the following alkenes with the first being the most stable and last being the least stable is
(i)
(ii)
(iii)
(iv)
EASY
The correct order of stability for the following alkoxides is:
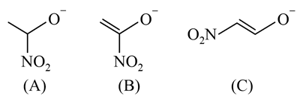
EASY
Resonance effect is not observed in
MEDIUM
Given below are two statements :
Statement I : Hyperconjugation is a permanent effect.
Statement II : Hyperconjugation in ethyl cation involves the overlapping of bond with empty orbital of other carbon.
Choose the correct option:
EASY
In pyrrole, the electron density is maximum on
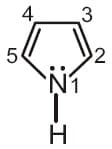
MEDIUM
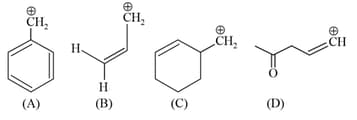
Among the given species the Resonance stabilised carbocations are:
EASY
Which of the following alkyl groups shows least positive inductive effect?
MEDIUM
Which one among the following resonating structures is not correct?
EASY
The following effect is known as___
EASY
Which of the following is not explained by hyperconjugation?
EASY
A tertiary butyl carbocation is more stable than a secondary butyl carbocation because of which of the following?
MEDIUM
In which of the following molecules, all atoms are coplanar?
EASY
Consider the following compounds
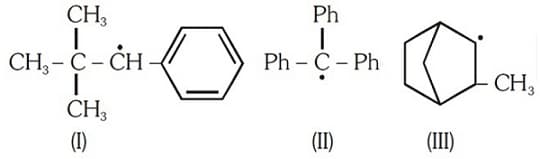
Hyperconjugation occurs in:

