EASY
Earn 100
How is relative density of a liquid determined by density bottle?
Important Questions on Physical Quantities and Measurement
MEDIUM
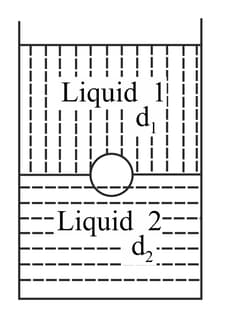
A jar is filled with two non-mixing liquids and having densities and , respectively. A solid ball, made of a material of density , is dropped in the jar. It comes to equilibrium in the position shown in the figure. Which of the following is true for , and ?
EASY
EASY
EASY
MEDIUM
EASY
While determining the density of the material of body a student recorded the following observations:
(i) Mass of the body
(ii) Reading of water level in the measuring cylinder without body
(iii) Reading of water level in the measuring cylinder with body . Based on these observations, the density of material of the body in is
MEDIUM
MEDIUM
EASY
i. Noted the water level in the measuring cylinder without the copper piece.
ii. Immersed the copper piece in the water.
iii. Noted the water level in the measuring cylinder with the copper piece inside it.
iv. Removed the copper piece from the water and immediately weighed it using a spring balance.
The wrong step in the procedure is
HARD
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
EASY
EASY
MEDIUM

