EASY
Earn 100
How many electrons are there in a molecule of ?
Important Questions on Structure of Atom
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
Write the name of particle present at the center of electrons in represented Rutherford's model. Write one characteristic of this model.
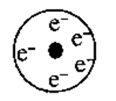
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
Write the name of the particle present in the sphere of represented Thomson atomic model. Explain the model with one example.

MEDIUM
EASY
EASY

