MEDIUM
Earn 100
How will you explain alpha elimination reactions?
Important Questions on Organic Chemistry: Some Basic Principles and Techniques
MEDIUM
In which of the following pairs is more stable than
EASY
Aldehydes or ketones when treated with the product formed is
EASY
The correct statement regarding electrophiles is:
MEDIUM
The increasing order of nucleophilicity of the following nucleophiles is;
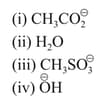
HARD
A solution of in toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of :
MEDIUM
The major product of the following reaction is:
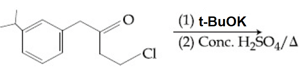
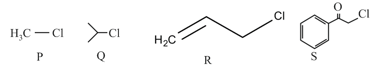
HARD
KI in acetone, undergoes SN2 reaction with each of P, Q, R and S. The rates of the reaction vary as
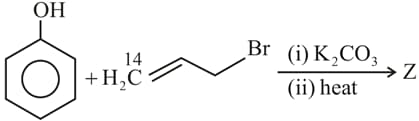
MEDIUM
In the following reaction sequence, structures of and are, respectively
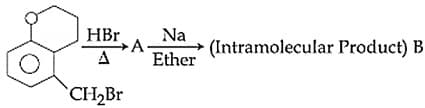
MEDIUM
In which of the following compounds, the bond ionization shall give most stable carbonium ion?
EASY
The compound that will react most readily with gaseous bromine has the formula:
HARD
The major product of the following reaction is
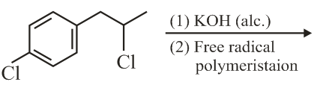
EASY
Which of the following statements is not correct for a nucleophile?
EASY
Which among the following is a set of nucleophiles?
MEDIUM
The compound that is most difficult to protonate is:
HARD
The major products and in the following reactions are
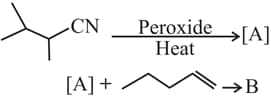
EASY
The lower stability of ethyl anion compared to methyl anion and the higher stability of ethyl radical compared to methyl radical, respectively, are due to
MEDIUM
The correct sequence of reagents for the following conversion will be
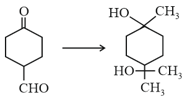
EASY
The order of stability of the following carbocations:
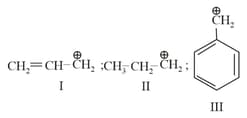
EASY
In an reaction on chiral centres, there is: