Hydrocarbon [], on reaction with boron hydride followed by treatment with yields []. On reductive ozonolysis of [] yields a mixture of two aldehydes [] and []. Of these, only [] can undergo Cannizarro reaction. [] exists in two geometrical isomers [] and [], of which [] is more stable. Give structures of [], [], [] [], [] and [] with proper reasoning.

Important Questions on Purification of Organic Compounds (Qualitative and Quantitative)
An organic compound, , was subjected to a series of tests in the laboratory. It was found that this compound does not react with but liberates hydrogen gas with sodium. It rotates a plane polarised light and also gives positive iodoform test. Draw the possible structure of the compound.
An optically active alcohol A, , was subjected to a series of tests in the laboratory. It was found that this compound reacts with Lucas reagent in about five minutes to give B. Give structures of A and B with proper reasoning and draw Fischer projections for A. Give reaction for the steps whereas possible.
and , two isomeric compounds with the molecular formula behave in the following ways.
can be easily oxidised to an acid whose calcium salt when distilled forms pentanone.
and , two isomeric compounds with the molecular formula behave in the following ways.
when treated with iodine and sodium hydroxide forms crystals of iodoform. Assign structures to and . Is there any other isomeric compound to these?
Identify A from the given set of reactions:
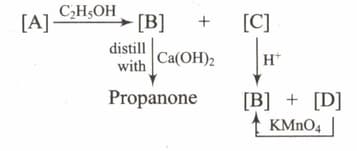
Identify the final product.
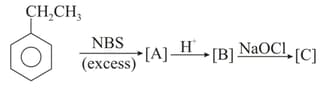
The original compound is
