Hydrogen bromide is a compound of the two elements hydrogen and bromine. It melts at and boils at . It has the same type of bonding as hydrogen chloride. Which type of bond is formed between the hydrogen and bromine atoms, in hydrogen bromide?

Important Questions on Atoms Combining
Hydrogen bromide is a compound of the two elements hydrogen and bromine. It melts at and boils at .
i. Name two other compounds with bonding similar to that in hydrogen bromide.
ii. Write formulae for these two compounds.
Aluminium and nitrogen react to form an ionic compound called aluminium nitride. These show the electron arrangement for the two elements:
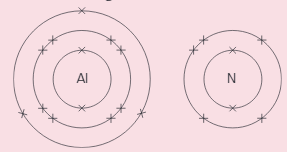
Answer these questions for an aluminium atom.
i) Does it gain or lose electrons, to form an ion?
ii) How many electrons are transferred?
iii) Is the ion formed positive, or negative?
iv) What charge does the ion have?
Aluminium and nitrogen react to form an ionic compound called aluminium nitride. These show the electron arrangement for the two elements:
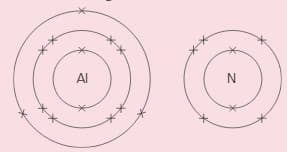
Answer these questions for a nitrogen atom.
i Does it gain or lose electrons, to form an ion?
ii How many electrons are transferred?
iii Is the ion formed positive, or negative?
iv What charge does the ion have?
Aluminium and nitrogen react to form an ionic compound called aluminium nitride. These show the electron arrangement for the two elements:
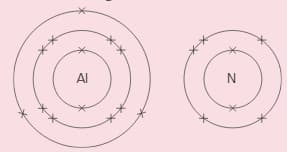
i) Give the electron distribution for the ions formed by the two atoms. ()
ii) What do you notice about these distributions? Explain it.
Aluminium and nitrogen react to form an ionic compound called aluminium nitride.
Name another non-metal that will form an ionic compound with aluminium, in the same way as nitrogen does.
The compound zinc sulphide has a structure like this:
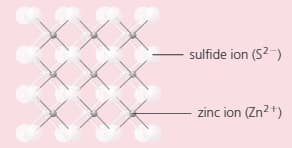
Which does the diagram represent: a giant structure, or a molecular structure?
