HARD
12th Tamil Nadu Board
IMPORTANT
Earn 100
Identify the Lewis acid and the Lewis base in the following reactions.
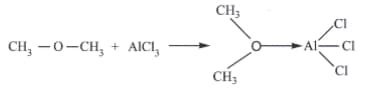


Important Questions on Ionic Equilibrium
HARD
12th Tamil Nadu Board
IMPORTANT
accepts hydroxide ion from water as shown below
Predict the nature of using Lewis concept
MEDIUM
12th Tamil Nadu Board
IMPORTANT
MEDIUM
12th Tamil Nadu Board
IMPORTANT
HARD
12th Tamil Nadu Board
IMPORTANT
HARD
12th Tamil Nadu Board
IMPORTANT
HARD
12th Tamil Nadu Board
IMPORTANT
MEDIUM
12th Tamil Nadu Board
IMPORTANT
HARD
12th Tamil Nadu Board
IMPORTANT
