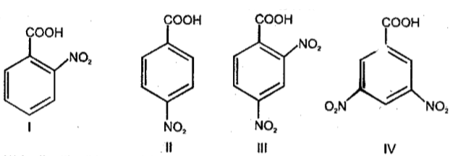
Identify the correct order of boiling points of the following compounds
(I)
(II)
(III)
Important Questions on Aldehydes, Ketones and Carboxylic Acids
Given below are two statements: one is labelled as Assertion and the other is labelled as Reason.
Assertion: A mixture contains benzoic acid and napthalene. The pure benzoic acid can be separated out by the use of benzene.
Reason: Benzoic acid is soluble in hot water.
In the light of the above statements, choose the most appropriate answer from the options given below.
Two statements are given belwo :
Statement I : The melting point of monocarboxylic acid with even number of carbon atoms is higher than that of with odd number of carbon atoms acid immediately below and above it in the series.
Statement II : The solubility of monocarboxylic acids in water decreases with increase in molar mass.
Choose the most appropriate option :
Statement (a): Carboxylic acids with and above are insoluble in water.
Statement (b): Ethanoic acid is having less boiling point than .
The correct answer is
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Hydroxybenzoic acid has a lower boiling point than hydroxybenzoic acid.
Hydroxybenzoic acid has intermolecular hydrogen bonding.
Decreasing order of melting point of compound to follows: