Identify whether the given compound is an element, a compound or a mixture:
Sugar solution
Important Questions on Is Matter around Us Pure?
(R = 8.314 J/mol K) (ln7.5 = 2.01)
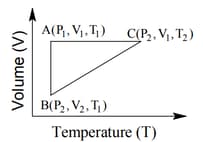
The three processes in a thermodynamic cycle shown in the figure are : Process is isothermal; Process is isochoric (volume remains constant); Process is adiabatic.
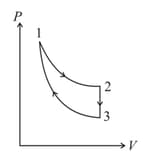
The total work done by the ideal gas in this cycle is, The internal energy decreases by, in the isochoric process. The work done by the gas in the adiabatic process is, . The heat added to the system in the isothermal process is
The combination of plots which does not represent isothermal expansion of an ideal gas is
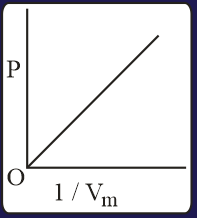
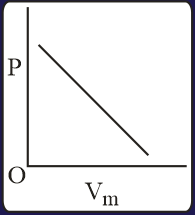
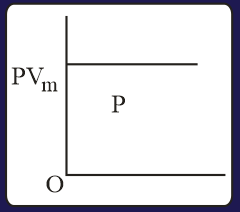
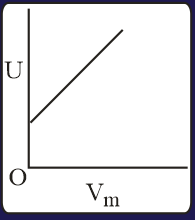
The correct option(s) is (are)
(Latent heat of ice is and )
[Heat of fusion of ice ; Specific heat of water ]
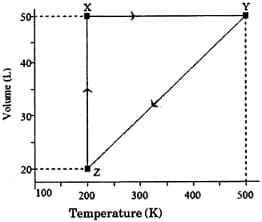
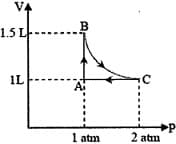
The pressure of the gas (in atm) at and respectively, are
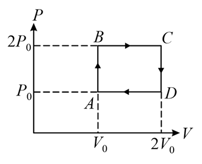
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
Select the correct options from the following statements:
I. and are isobars of each other.
II. reacts with to form a product which contains ionic bonds.
III. and are isobars of each other.
IV. reacts with to form a compound whose aqueous solution is known as lime water.

