MEDIUM
Earn 100
If is the initial concentration of the reactant, the half-life period of the reaction of order is proportional to which of the following?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
HARD
The activation energy of the backward reaction exceeds that of the forward reaction by (in ). If the pre-exponential factor of the forward reaction is times that of the reverse reaction, the absolute value of for the reaction at is ____.
(Given; and is the Gibbs energy)
HARD
For an elementary chemical reaction, , the expression for is:
HARD
(Assume that all these gases behave as ideal gases)
HARD
MEDIUM
EASY
MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
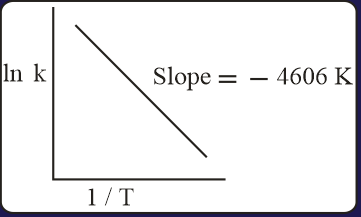
MEDIUM
[Gas constant, ]
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

EASY
EASY
Rate
If the concentration of A is kept the same but that of B is doubled what will happen to the rate itself?
HARD
EASY
EASY
MEDIUM
| Initial Concentration (A) | Initial Concentration (B) | Initial rate of formation of C |
|---|---|---|
The rate law for the formation of is
EASY
EASY
MEDIUM
Which one of the following statements is correct?
EASY
EASY

