MEDIUM
Earn 100
If 50 glucose molecules combine to form 'A' polysaccharide. The general formula of 'A' polysaccharide will be
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Biomolecules
MEDIUM
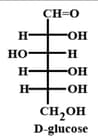
The correct structure(s) of glucopyranose is(are)
EASY
MEDIUM
Aldehyde as carbonyl group
MEDIUM
EASY
MEDIUM
Sucrose is not:
MEDIUM
Five alcohol groups
EASY
EASY
EASY
Assertion (A) : Sucrose is a disaccharide and a non-reducing sugar.
Reason (R): Sucrose involves glycosidic linkage between of -glucose and of -fructose.
Choose the most appropriate answer from the options given below :
EASY
MEDIUM
A) In a polysaccharide chain, the right end is reducing end and the left end is non-reducing end.
B) In a protein, the right end is a first amino acid called N-end, and the left end is called C-terminal.
C) Concanavalin A is a drug used to cure cancer along with vinblastin and curcumin.
D) GLUT- enables glucose transport into cells.
EASY
MEDIUM
Amylose and Amylopectin
EASY
HARD
Define optical activity. How many optical isomers are possible for glucose.
HARD
HARD
| List-I | List-II | List-III | |||
| (A) | Deoxyribose | (i) | Gums | (I) | Fatty acids |
| (B) | Lipids | (ii) | (II) | True macromolecules | |
| (C) | Polymeric substances | (iii) | Trypsin | (III) | Cellulose |
| (D) | Polypeptides | (iv) | Glycerol | (IV) | Carbohydrate |
The correct match is:
EASY
EASY

