EASY
Earn 100
If 500ml of gas A at 400 torrs and 666.6 ml of B at 600 torrs are placed in a 3 liter flask, the pressure of the system will be
(a)200 torr
(b)366 torr
(c)550 torr
(d)100 torr
50% studentsanswered this correctly
Important Questions on States of Matter
MEDIUM
MEDIUM
HARD
(Given: The vapour pressure of water at is )
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
EASY
[Use atomic masses (in ): ]
EASY
MEDIUM
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?
HARD
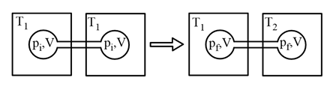
HARD
HARD
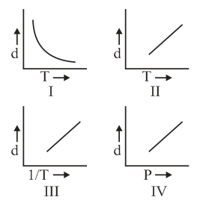
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
EASY
The above reaction is carried out in a vessel starting with partial pressure , and . When the reaction is complete, the total pressure in the reaction vessel is _____ .
(Round off of the nearest integer).
HARD
EASY

