EASY
NEET
IMPORTANT
Earn 100
If and solution is example of non-ideal solution then which graphical representation is correct?
(a)
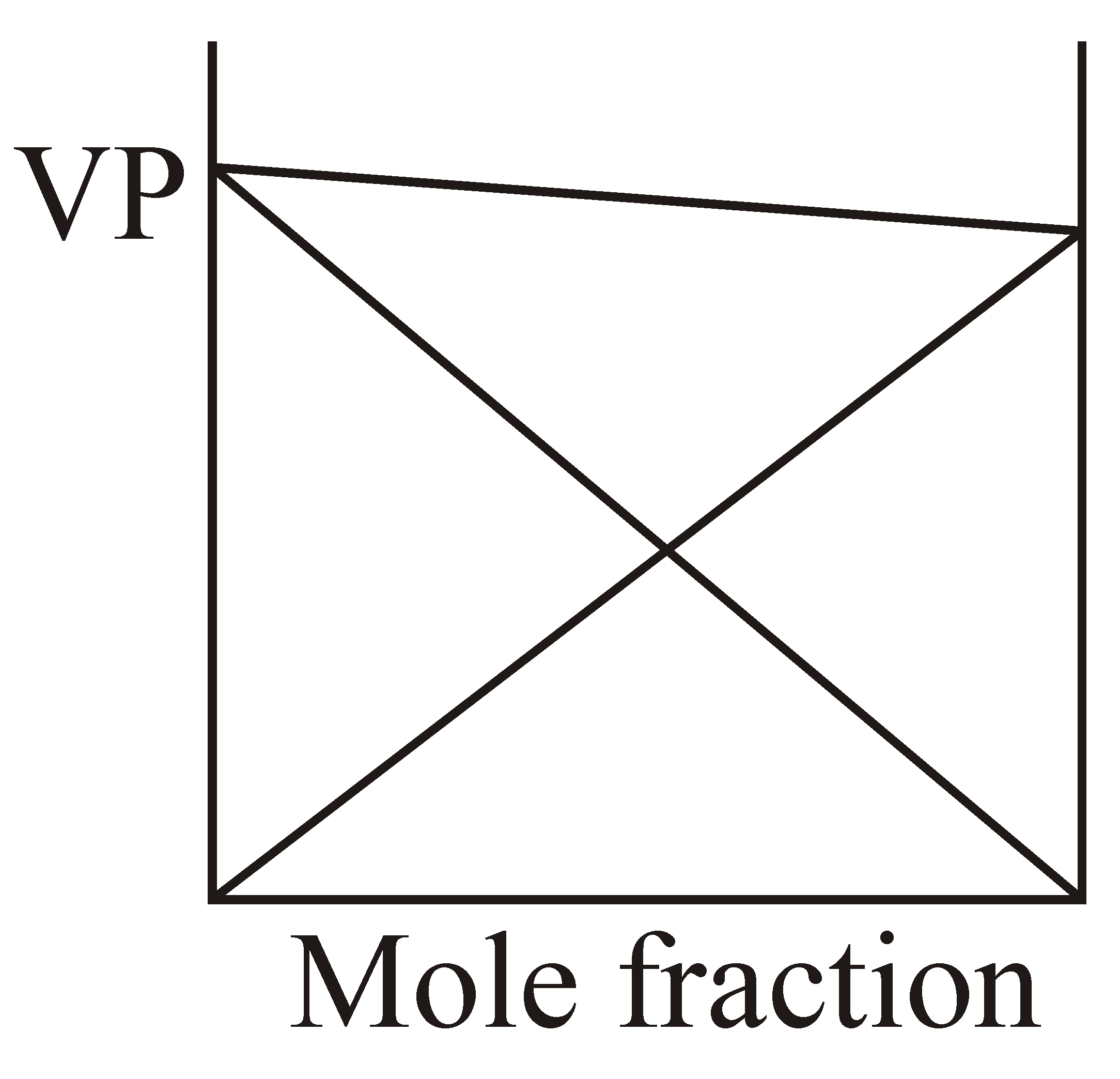
(b)
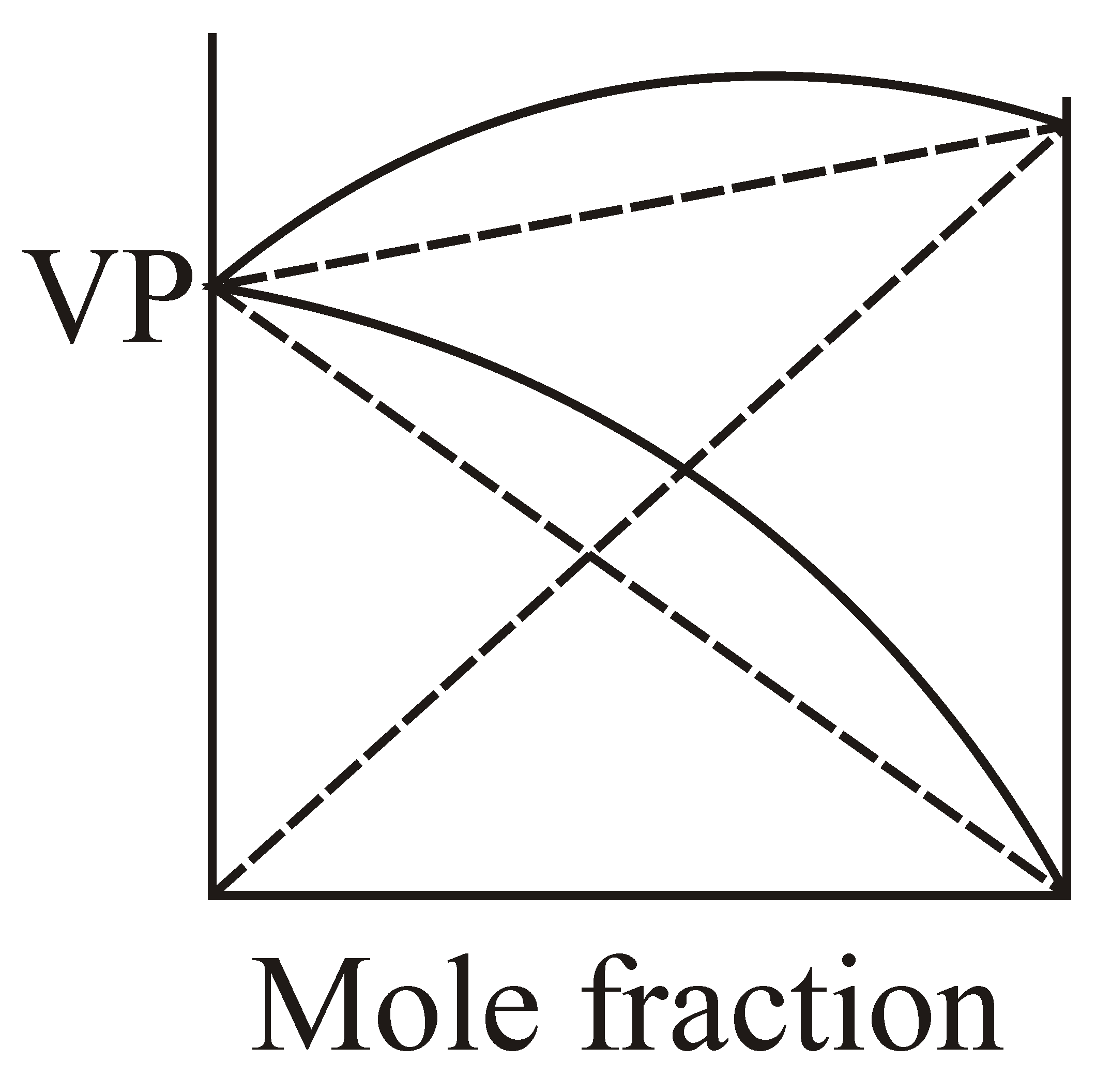
(c)
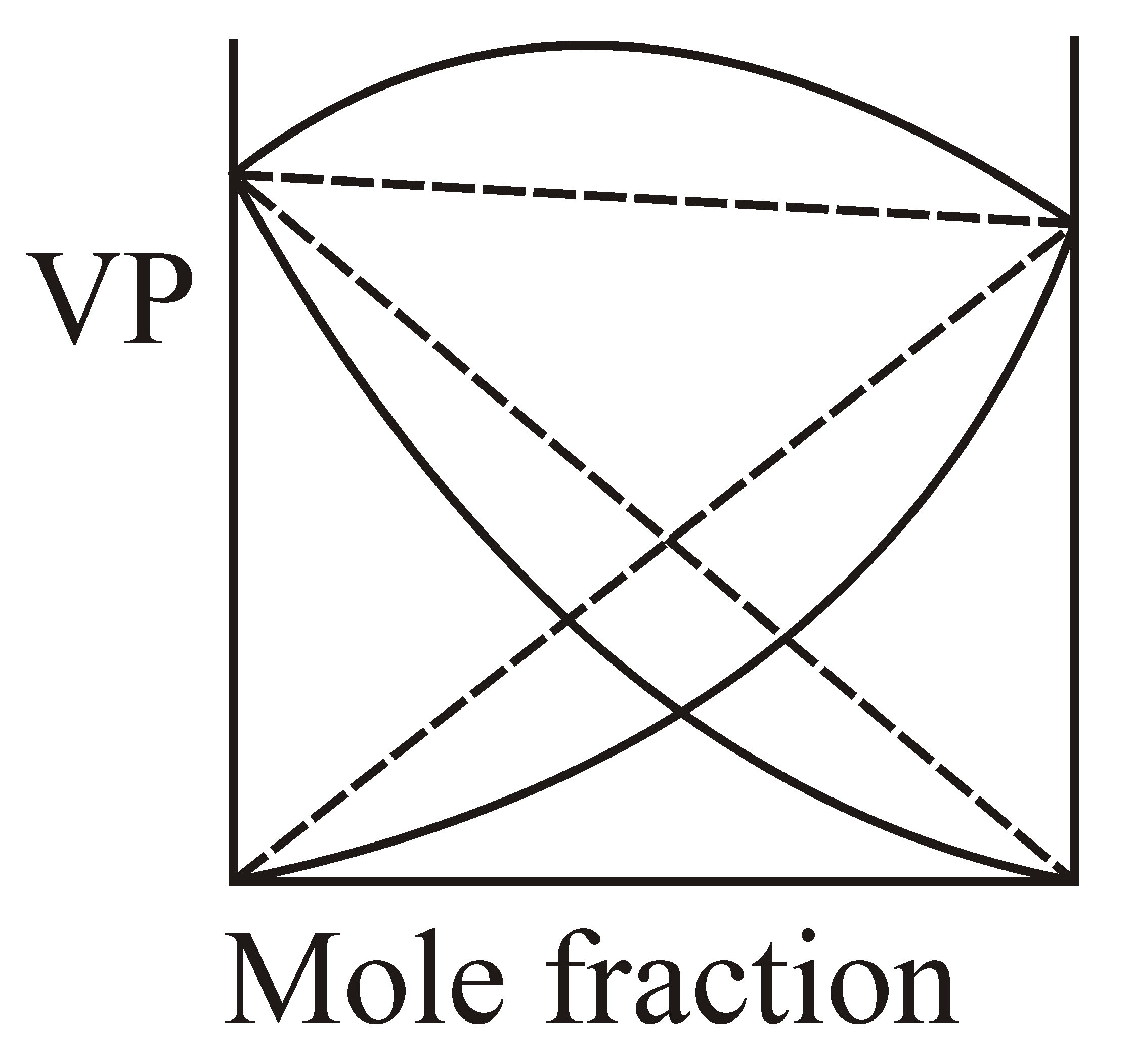
(d)
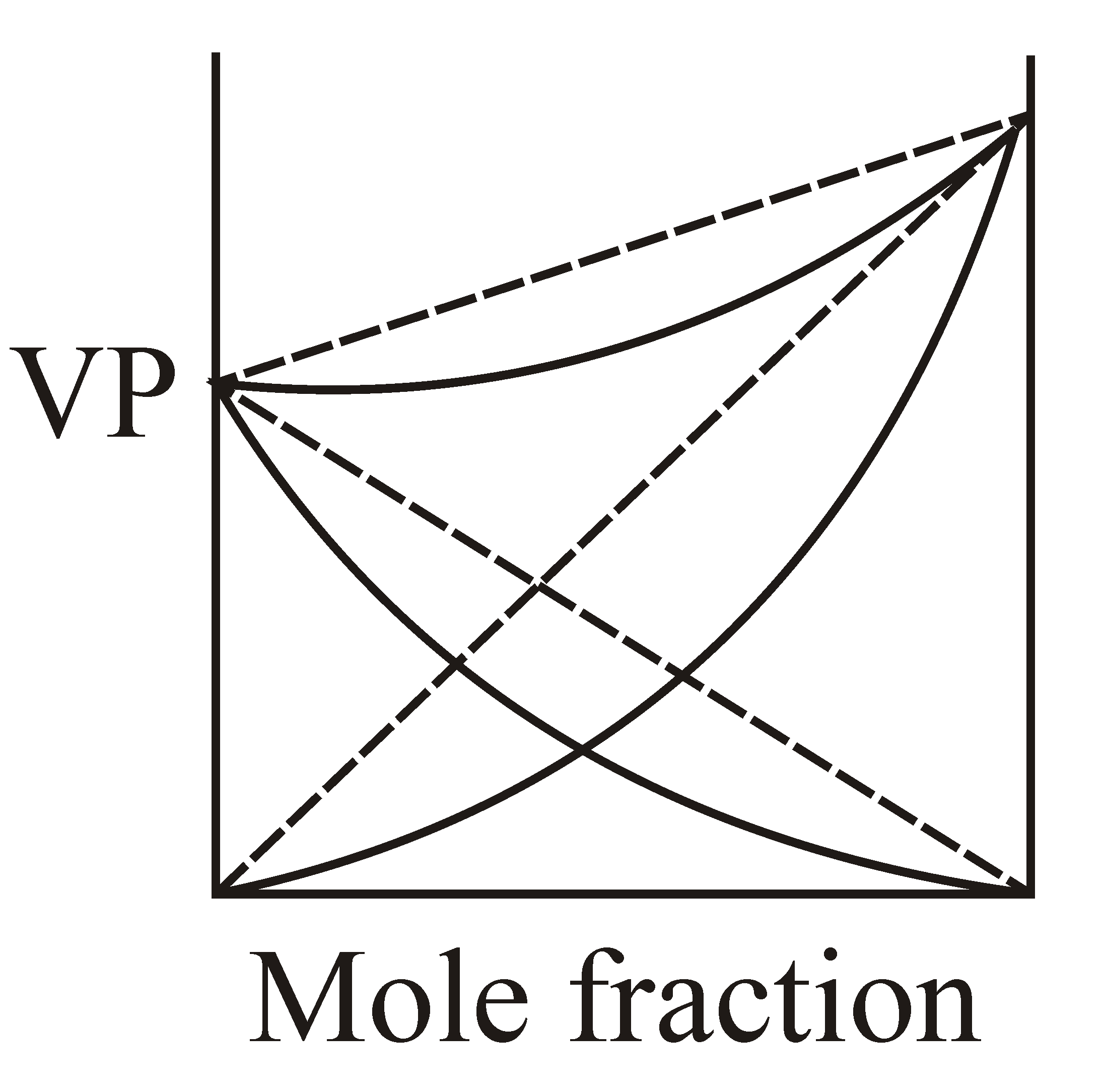
100% studentsanswered this correctly

Important Questions on Solutions
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
