If both the functional groups of salicylic acid, , ionise in water with for the group and for the group, calculate of the saturated solution of the acid (solubility ).

Important Questions on Miscellaneous Problems for Revision
The density of trifluoroacetic acid vapour was determined at and and was found to be . Calculate for:
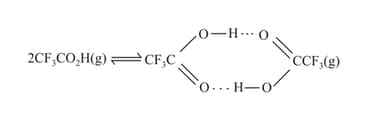
The following equilibrium exist simultaneously in a vessel.
If initially only and are present in ratio and the total pressure at equilibrium is and the partial pressure of is calculate the equilibrium partial pressure of and .
In a reaction of the type:
the equilibrium concentrations of and are and respectively. Argon is then introduced at equilibrium at constant volume. Calculate the concentrations of and at the new equilibrium positions.
The rate law of the reaction given below is given as product.
Rate
Find .
Nitric acid is prepared from ammonia in a three-step process:
(i) (fast)
(ii) (slow)
(iii) (fast)
Calculate how much can be produced from of ammonia assuming efficiency in each of the reactions.
If the answer is of type , report the value of correct up to two places of decimals.
If given chemical reaction is a second-order in and first-order in , calculate the rate of formation of when oxygen concentration is and the nitric oxide concentration is
When an electron in an excited molybdenum atom falls from shell, an X -ray is emitted. These X-rays are diffracted at an angle of by planes with a separation of . What is the difference in energy in joules between the shell and the shell in assuming a first-order diffraction?
If the answer is of type , report the value of correct up to two places of decimals.
