MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
If decomposition reaction follows first order kinetics then the graph of rate conformation of against time t will be:
(a)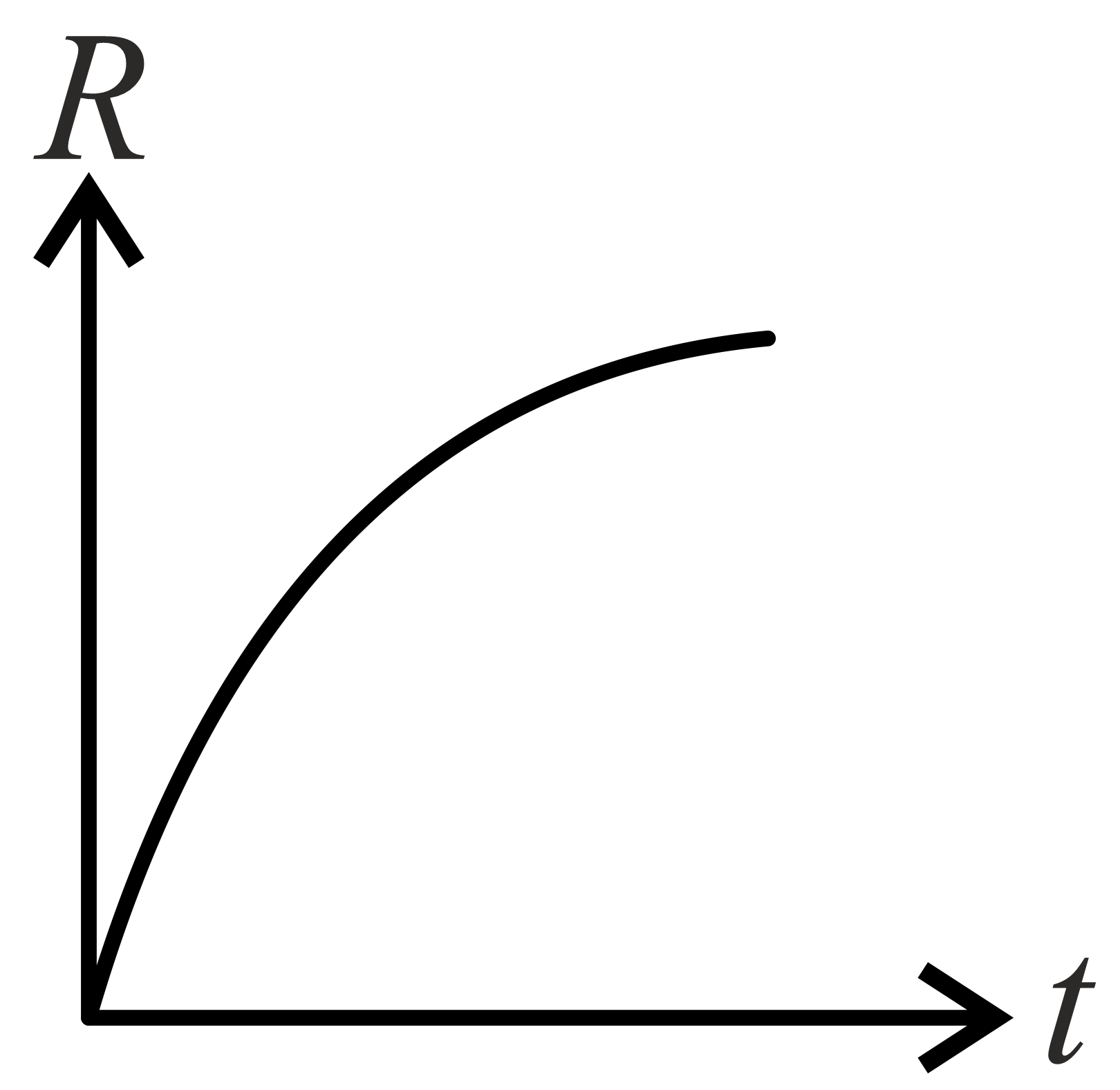

(b)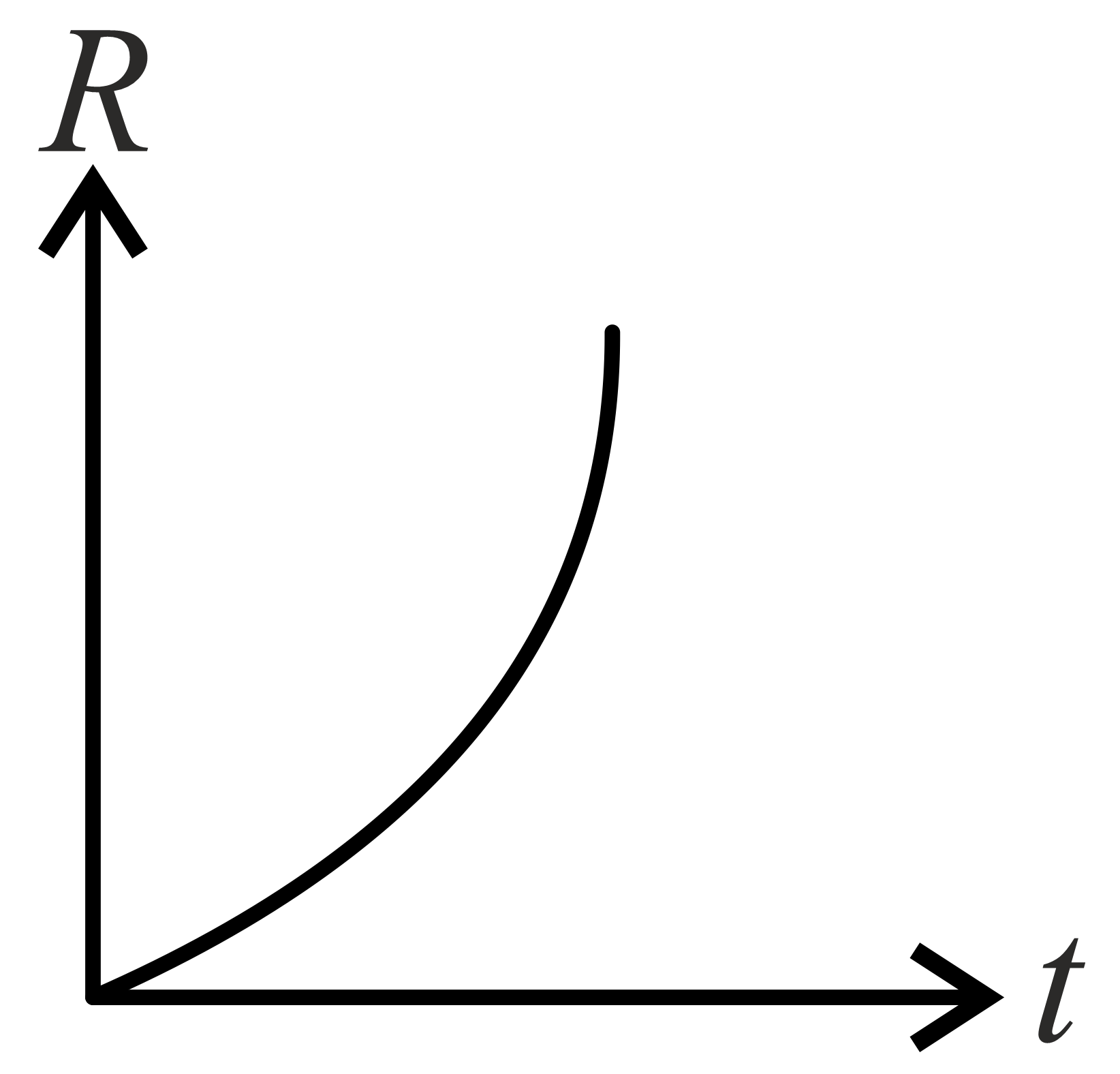

(c)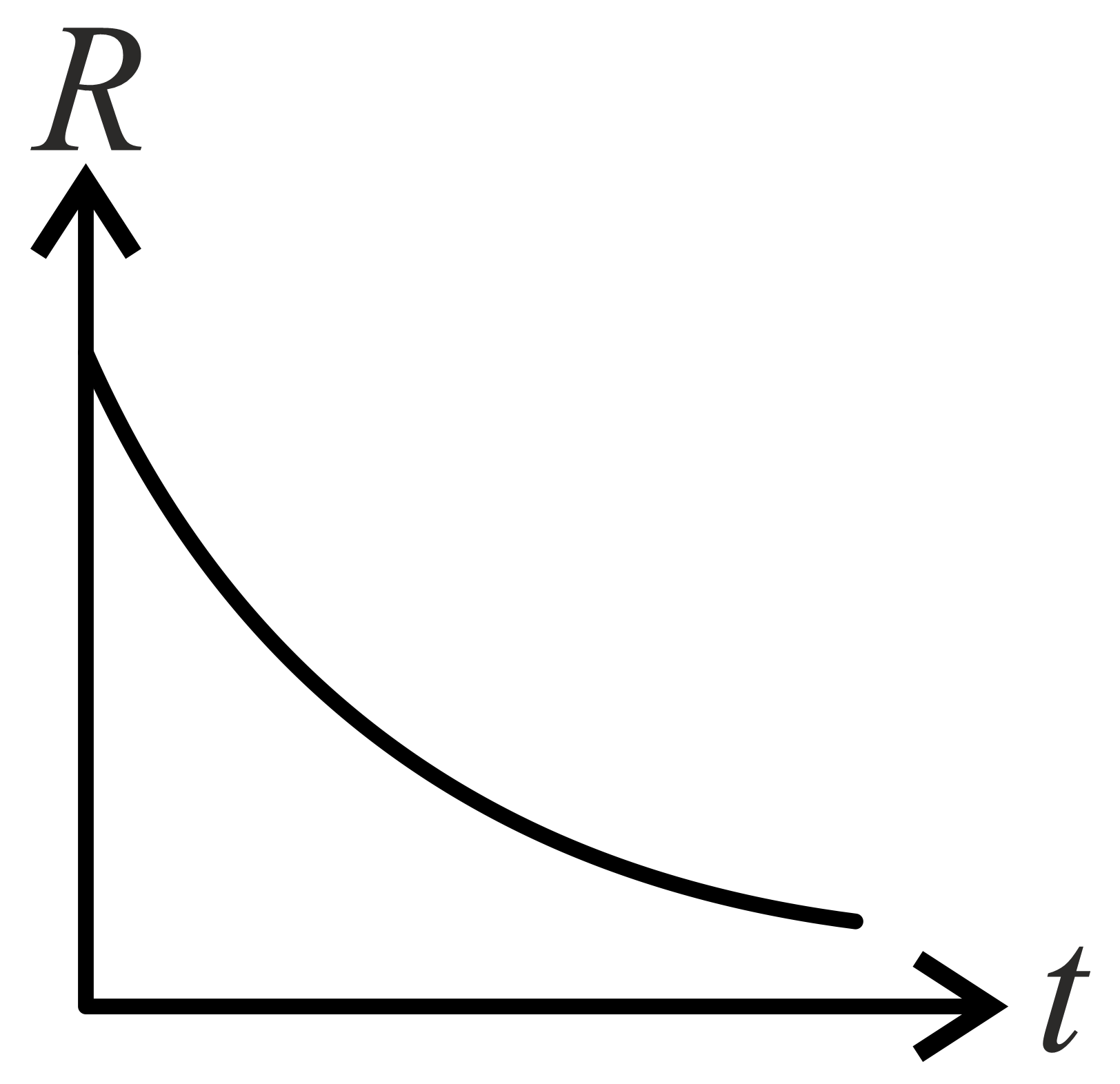

(d)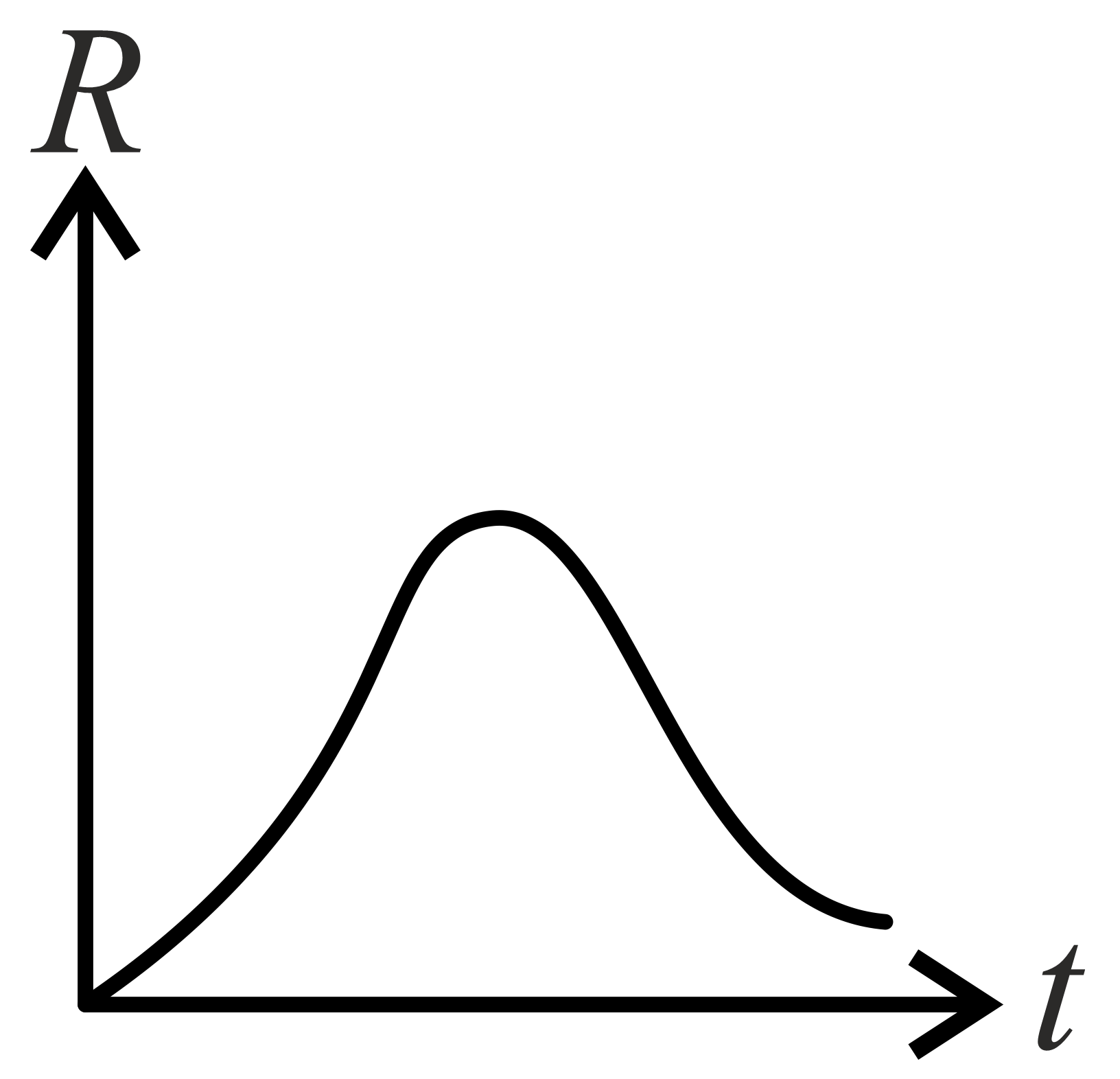

44.44% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main/Advance
IMPORTANT
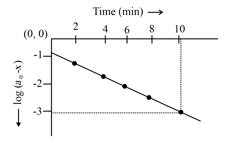
For the first order decomposition of ,
a graph of vs is shown in figure. What is the rate constant ?
MEDIUM
JEE Main/Advance
IMPORTANT
Consider the reaction , graph between half life and initial concentration (a) of the reactant is
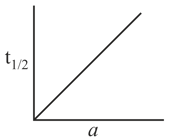
Hence, graph between And time will be
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
Consider the following first order competing reactions :
and
If of the reaction of was completed when of the reaction of was completed, the ratio of their rate constants is :
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
Consider this reaction.
The reaction of nitrogen dioxide and ozone represented in first order in and . Which of these possible reaction mechanisms is consistent with the rate law?
EASY
JEE Main/Advance
IMPORTANT
