EASY
NEET
IMPORTANT
Earn 100
If degree of dissociation of is in a solvent, then
(a)Normal boiling point = experimental boiling point
(b)Normal osmotic pressure experimental osmotic pressure
(c)Normal molecular weight experimental molecular weight
(d)Normal freezing point experimental freezing point
75% studentsanswered this correctly

Important Questions on Solutions
EASY
NEET
IMPORTANT
Vapour phase diagram for a solution is given below if dotted line represents deviation
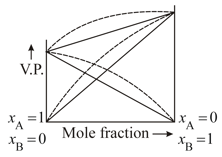
Correct observation for this solution
EASY
NEET
IMPORTANT
Vapour pressure diagram of some liquids potted against temperature are show below
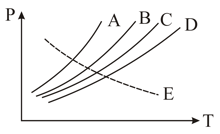
Most volatile liquid
EASY
NEET
IMPORTANT
Two solutions marked as and are separated through semipermeable membrane as below. The phenomenon undergoing
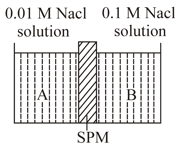
EASY
NEET
IMPORTANT
Correct observation
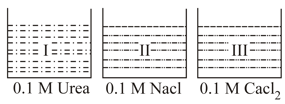
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
The phenomenon taking place
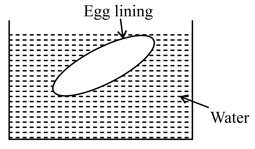
EASY
NEET
IMPORTANT
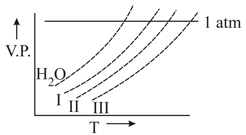
Which is having highet elevation in boiling point?
EASY
NEET
IMPORTANT
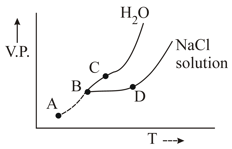
Freezing point of solution is marked as
