MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
If each of the following salts has solubility product which of them is least soluble in water?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Equilibrium
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
When a weak base solution is titrated with a strong acid the of solution initially decreases fast and then decreases slowly till near equivalence point (as shown in the Which of the following statements is/are true?
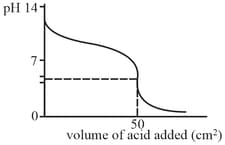
MEDIUM
JEE Main/Advance
IMPORTANT
A solution of a substance is titrated against a strong base (or acid), volume of the strong base (or acid) is plotted against of the solution (as shown in the figure). The substance could be:
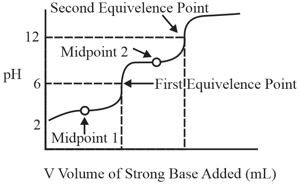
HARD
JEE Main/Advance
IMPORTANT
Select the correct graph(s) for the corresponding acid-base titration:
HARD
JEE Main/Advance
IMPORTANT
