MEDIUM
Earn 100
If rate constant at temperature and rate constant at temperature for a first-order reaction, then which of the following relation is correct?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
EASY
MEDIUM
MEDIUM
(Assume Activation energy and pre-exponential factor are independent of temperature; )
MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
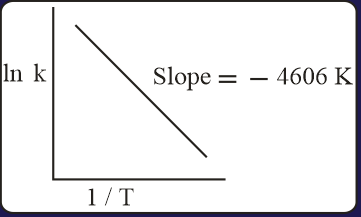
MEDIUM
MEDIUM
MEDIUM
Identify the incorrect statement.
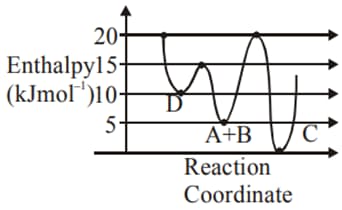
EASY
EASY
MEDIUM
The Arrhenius plots of two reactions, I and II are shown graphically-
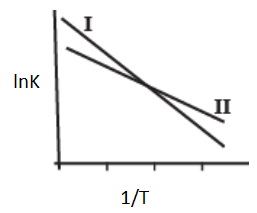
The graph suggests that-
EASY
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
[Gas constant, ]
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

HARD

