EASY
JEE Main/Advance
IMPORTANT
Earn 100
If molar heat capacity of a gas (monoatomic) in a certain process is , if amount of heat is given to this gas, then rise in internal energy of gas is given by
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
Heat is supplied by heater so that piston of mass moves a distance in upward direction and achieve a speed then work done by gas is given by (initially spring was in its natural length, : area of cross section)

EASY
JEE Main/Advance
IMPORTANT
A cyclic process is shown in figure.
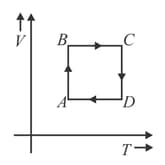
Which of the following statements is correct?
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
An ideal gas is enclosed in a thermally insulated container. Container is covered by an insulating piston which is connected with spring. Small displacement is given to piston as shown in figure. Which of the following statements is correct? Neglect friction everywhere.
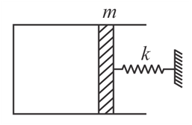
EASY
JEE Main/Advance
IMPORTANT
