EASY
NEET
IMPORTANT
Earn 100
If the rate of reaction increases to two times with every rise in temperature, then if we raise the temperature by , the new rate of the reaction will be
(a) times
(b) times
(c) times
(d) times
100% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
is . What is the activation energy for the backward reaction?
EASY
NEET
IMPORTANT
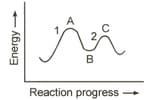
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
