In Rutherford's experiment, a thin gold foil was bombarded by alpha particles. If Thomson's "Plum Pudding Model" of the atom were correct, what would have been the outcome of the experiment?
Important Questions on Atoms
Scattering angle
Number of scattered -particles detected
(Plots are schematic and not to scale)
(Ignore radiation due to motion of electric charge)
Consider the following statement:
(I) All isotopes of elements have the same number of neutrons.
(II) Only one isotope of an element can be stable and non-radioactive.
(III) All elements have isotopes.
(IV) All isotopes of Carbon can form chemical compounds with Oxygen.
The correct option regarding an isotope is:
In Thomson's experiment for the determination of of electron, electric field of intensity only is applied. Now the deflection produced by the electron beam is directly proportional to:
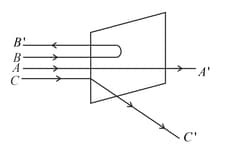
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
Match List (Experiment performed) with List (Phenomena discovered/associated)and select the correct option from the options given below the lists
| List-I | List-II | ||
|---|---|---|---|
| Davisson and Germer experiment | Wave nature of electrons | ||
| Millikan's oil drop experiment | Charge of an electron | ||
| Rutherford experiment | Quantisation of energy levels | ||
| Franck-Hertz experiment | Existence of the nucleus |

