HARD
Earn 100
In a cubic packed structure of mixed oxides, the lattice is made up of oxide ions, one fifth of tetrahedral voids are occupied by divalent ions, while one-half of the octahedral voids are occupied by trivalent ions , then the formula of the oxide is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on The Solid State
EASY
MEDIUM
HARD
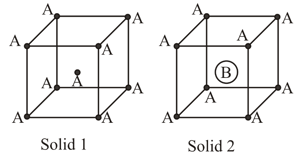
Consider the bcc unit cells of the solids and with the position of atoms as shown below. The radius of atom is twice that of atom The unit cell edge length is more in solid than in . What is the approximate packing efficiency in solid ?
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
The packing efficiency of the face centered cubic (FCC), body centered cubic (BCC) and simple/primitive cubic (PC) lattices follows the order
EASY
MEDIUM
EASY
The vacant space in bcc lattice unit cell is:
MEDIUM
MEDIUM
HARD
An element crystallises in a face–centred cubic (fcc) unit cell with cell edge a. The distance between the centres of two nearest octahedral voids in the crystal lattice is:
EASY
MEDIUM
EASY
EASY

MEDIUM

