HARD
Earn 100
In a double titration method, for the mixture of NaHCO3 and Na2CO3, the volume of HCl used at phenolphthalein indicator end point was x ml and further of Methyl orange indicator end point was y ml. The volume of HCl for complete reaction of NaHCO3 is -
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Equilibria
HARD
EASY
EASY
HARD
MEDIUM
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with $\mathrm{NaOH}$ phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below:
HARD
HARD
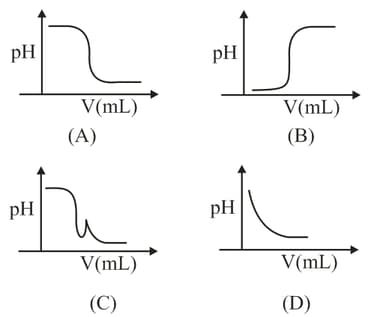
HARD
MEDIUM
MEDIUM
EASY
EASY
HARD
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
HARD
MEDIUM
MEDIUM
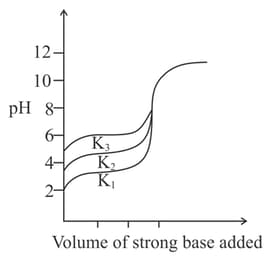
Titration curves for solutions of three weak acids and with ionization constants and respectively are plotted as shown in the figure. Which of the following is/are true?
HARD
MEDIUM
EASY
The titration curve for titration of a solution of a diprotic acid with is shown below.
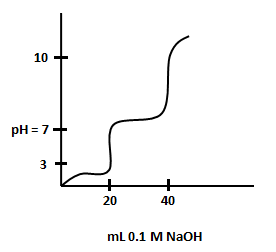
and are approximately

