MEDIUM
Earn 100
In a homonuclear molecule which of the following set of orbitals is degenerate?
(a)and
(b)
(c)and
(d)
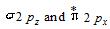
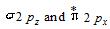
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
Stability of the species increases in the order of
MEDIUM
In which of the following pairs of molecules/ions, both the species are not likely to exist?
EASY
Which of the following is paramagnetic?
HARD
Decreasing order of stability of and is:
HARD
In which of the following processes, the bond order has increased and paramagnetic character has changed to diamagnetic?
MEDIUM
Which one of the following properties is not shown by NO ?
EASY
Which one of the following molecules is expected to exhibit paramagnetic behaviour?
MEDIUM
According to molecular orbital theory, which of the following molecule will not be available?
EASY
Which of the following species is not paramagnetic?
EASY
The diamagnetic species is:
EASY
After understanding the assertion and reason, choose the correct option.
Assertion: In the bonding molecular orbital (MO) of electron density is increased between the nuclei.
Reason: The bonding MO is , which shows destructive interference of the combining electron waves.
MEDIUM
The correct bond order in the following species is:
MEDIUM
Which of the following has unpaired electron(s)?
EASY
Which one of the following pairs of species has the same bond order?
MEDIUM
Which one of the following molecules is paramagnetic?
HARD
Among the following molecules/ ions,
Which one is diamagnetic and has the shortest bond length?
HARD
Assuming mixing is NOT operative, the paramagnetic species among the following is
MEDIUM
Consider the following species:
(i)
(ii)
(iii)
Among the following, sigma bond alone is present in -
HARD
Which of the following options represents the correct bond order?
MEDIUM
Two pi and half sigma bonds are present in:

