In an adiabatic process, of work is done on the gas. The change in internal energy of the gas

Important Questions on Kinetic Theory of Gases and Radiations
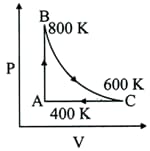
One mole of diatomic ideal gas undergoes a cyclic process as shown in the figure. The process is adiabatic. The temperatures at and are and , respectively. Choose the correct statement.
The internal energy change in a system that has absorbed of heat and done of work is similar,
If denote respectively the heat added, change in internal energy and the work done in a closed cycle process, then,
of heat is added to a gaseous system, whose internal energy change is , then the amount of external work done is,
When heat energy of is supplied to a gas at constant pressure there is an increase in its volume equal to . The increase in internal energy of the gas in is
During an isothermal expansion, a confined ideal gas does of work against its surrounding. This implies that,
If the amount of heat given to a system is and the amount of work done on the system is then the change in internal energy of the system is,
