HARD
Earn 100
In an open economy, which component of aggregate demand accounts for goods and services sold to other countries?
(a)Exports
(b)Consumption
(c)Investment
(d)Government Expenditure
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
Give a diagrammatic representation of energy flow through different trophic levels.
EASY
Explain the role of the ‘first trophic level’ in an ecosystem.
MEDIUM
Explain energy flow in ecosystem.
MEDIUM
A first order reaction is found to have a rate constant, . Find the half life of the reaction.
EASY
Sulphur sol is a type of
HARD
Derive an integrated rate equation for rate constant of a first order reaction.
MEDIUM
Describe the DDT biomagnification occurring in an aquatic food chain. State the negative effects the process has on the organisms at the last trophic level of the food chain.
EASY
The decomposition of ammonia on a platinum surface is a zero-order reaction. If the rate constant is , how long will it take to reduce the initial concentration of ammonia from .
EASY
If 20J of energy is trapped at producer level, then how much energy will be available to peacock as food in the following chain?
plant mice snake peacock
MEDIUM
How is the detritus food chain connected with the grazing food chain in a natural ecosystem?
EASY
Derive an integrated rate equation for a first order reaction.
HARD
The following graph depicts the inverse of magnification versus the distance between the object and lens data for a setup. The focal length of the lens used in the setup is
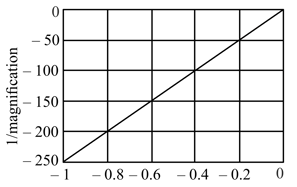
Distance between the object and the lens
EASY
A first order reaction takes minutes for decomposition. Calculate ?
MEDIUM
The given plots represent the variation of the concentration of a reactant with time for two different reactions . The respective orders of the reaction are
(i) 
(ii) 
EASY
The rate constant for a first order reaction is How much time will it take to reduce the initial concentration of the reactant to its value?
HARD
In a reaction, the initial concentration of the reactants increases fourfold and the rate becomes eight times its initial value. The order of the reaction is -
MEDIUM
A first order reaction is completed in minutes at and in minutes at . Calculate the activation energy of the reaction. (Given : )
EASY
What is a trophic level in an ecosystem ? What is ‘standing crop’ with reference to it?
EASY
Which of the following statement is NOT correct about solution?
HARD
For a concave lens of focal length , the relation between object and image distance and respectively, from its pole can best be represented by ( is the reference line):

